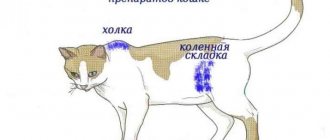
Owners of cats that are fed a natural diet should definitely think about which source of calcium to choose for their pet. The problem is that mainly phosphorus enters the body with meat: it contains minimal amounts of calcium.
At the same time, the balance of calcium and phosphorus in cats’ food should be 1.2:1 or at least 1:1, for kittens – 1.4:1. If you ignore this rule, you can create conditions for the development of serious diseases, such as secondary hyperparathyroidism and others. Therefore, calcium should be supplied with food regularly. Where can I get it?
Bones with meat
The classic source of calcium for cats, according to the postulates of the natural nutrition system, is bones. But there are rules here. If they are not followed, you can cause the cat to have a blockage in the intestines or even perforation of the walls of the mucous membrane, followed by surgery or death. Unfortunately, even animals that are brought to the operating table in time with such a problem cannot always be saved.
In view of this reason, many owners choose a different path for themselves - not to feed bones at all, in order to reduce the risks to zero. But then you will have to take calcium from other sources. Let us say right away that cats cannot absorb calcium from products of plant origin.
Eggshell: calcium carbonate
Of the natural sources of calcium, eggshells immediately come to mind. Indeed, it consists of 36% calcium. But there is one subtlety: the shell contains calcium carbonate, a compound that is only 20% absorbed. Thus, in order to maintain a balance of calcium and phosphorus of 1.2:1 for adult cats and 1.4:1 for kittens, a lot of shells are needed.
To calculate how many shells a cat needs per day, the daily meat consumption is taken into account. Let's say a cat eats 200 grams of veal per day, which corresponds to about 500 mg of phosphorus and about 50 mg of calcium. The amount of phosphorus depends on the type of meat (see table below). Accordingly, such a daily dose should contain at least 450 mg of calcium. To collect this amount, you need to take 1-1.5 grams of shell, which corresponds to 1 quail egg shell, because... its weight is approximately 1.5 grams. But you need to remember that calcium carbonate will not be absorbed entirely, but at best only 20%. That is, this is already about 5-7 grams per 200 grams of food per day. This mass corresponds to approximately 3-4 quail shells or 1-1.5 chicken shells.
But the fact that you add shells to food does not guarantee that calcium will be absorbed by at least 20%, because the shell still needs to be crushed into powder: otherwise, in many cats it comes out in pieces along with the feces (the author of the article saw this with his own eyes).
To increase the amount of calcium absorbed, you cannot pour whole spoonfuls of shells into a plate, because calcium carbonate in large quantities causes side effects: it lowers the acidity of the stomach below normal, causes flatulence, constipation, allergic reactions, and promotes the formation of kidney stones. In addition, by receiving calcium in excess, the body further reduces its absorption.
Thus, in order to meet your cat’s calcium needs every day throughout his life with the help of shells, you need to work hard. First, the shells need to be crushed so that the cat eats them and does not leave them, and so that the shells are digested. Secondly, you need to clearly count the amount so as not to overdo it, but also not to give too little. Thirdly, not every cat’s body can absorb at least 20% of calcium carbonate. Therefore, it is advisable to combine the shell with other sources of this mineral or abandon it altogether.
Contraindications and side effects
It should not be given to cats if there is an excess of the microelement in the body. If your pet is allergic, then vitamin complexes must be selected with caution, because they quite often cause various reactions. It is not advisable to give medicine to cats if they have kidney disease, hyperparathyroidism and some malignant tumors. It is necessary to choose a complex for kittens with a veterinarian.
Side effects include allergic reactions, nausea, vomiting, and diarrhea. The cat may have a stomach ache and flatulence. Sometimes disorders of the nervous system and psyche appear. If the dosage is too high, hypercalcemia may develop. If your pet experiences any unwanted effects, you should notify your veterinarian. Most likely, the medicine will have to be discontinued.
Cottage cheese
Calcium for cats is also found in dairy products. However, milk cannot be digested by cats older than 3 months and can cause diarrhea. Cheese is a fairly heavy food for cats and should only be used as a treat. But in terms of calcium, cottage cheese is valuable.
Medium-fat cottage cheese contains 111 mg of calcium and 150 mg of phosphorus per 100 grams. But we remember that the balance of calcium and phosphorus in a cat’s food should be 1.2 (calcium) to 1 (phosphorus) or at least 1:1. Those. in cottage cheese this ratio is not maintained: the bias in favor of phosphorus, as in meat, is simply not as pronounced. And if we take into account that cottage cheese cannot form the basis of the diet and is used only as a small part of it, it turns out that with the help of cottage cheese alone the balance of calcium and phosphorus cannot be achieved. In addition, not all cats agree to eat cottage cheese.
However, this product can be additionally calcified - and such a move can indeed balance the diet. To do this, you need to purchase calcium supplements from a veterinary pharmacy and add them to cottage cheese or other products.
What's the benefit?
Calcium is responsible for the strength of bones and teeth, which is important for the development of the skeleton and the full life of a cat. The microelement is necessary during the formation of a growing organism. It is involved in metabolism and blood circulation, is responsible for strengthening the heart, nerves, blood vessels, and helps the British and other breeds in successful fertilization, during pregnancy, and childbirth.
The normal level of calcium in the blood of cats is 2.0-2.7 mmol/l.
Its deficiency leads to various pathological conditions:
- Bones and teeth become brittle and break quickly.
- The pet experiences body tremors and heart dysfunction.
- Convulsions occur.
- In young animals, bones do not form properly.
- Rickets develops.
Cat food with calcium
Any food of at least premium class contains the required proportion of calcium and phosphorus. Calcium may not be present in the most easily digestible form, but it is there nonetheless. If your cat eats a high-quality food (preferably super-premium or holistic) and has good digestion, there is no need to worry about a lack of calcium.
Just choose food according to age. Since calcium is especially necessary for kittens (they develop their skeletal system), manufacturers have specially developed food for babies with a high content of this mineral. Therefore, it is worth looking at special food for kittens with calcium, and not immediately putting them on an adult product.
Calcium supplements for cats
Below we will talk about calcium supplements, which can be bought in human or veterinary pharmacies, as well as in pet stores. Calcium for cats is sold separately in tablets, as well as calcium as part of vitamin and mineral complexes.
To choose the right supplement for your cat, there are a few factors to consider.
- If for some reason the cat is on a diet using boiled meat rather than raw meat, then its diet is depleted not only of calcium, but also of other microelements, many of which are destroyed during heat treatment. Therefore, you can choose a supplement consisting not only of calcium, but entire vitamin and mineral complexes, which contain all the beneficial substances in the required balance. Just keep in mind that hypervitaminosis is much worse than hypovitaminosis: you cannot give such supplements every day throughout your life, and even by eye - strictly follow the instructions or prescription of the veterinarian.
- If the cat is fed raw meat without a bone component, then only calcium needs to be added to the food; other microelements and vitamins may be required only when indicated or during molting. Choose supplements that contain calcium and do not include phosphorus.
- When choosing a calcium supplement, you should also take into account the cat’s individual reaction. Some calcium supplements may not be suitable (this will manifest itself, for example, in an allergic reaction).
- If the animal is on fortified factory feed, supplements are contraindicated.
Calcium supplements are created from various natural products: shells, chitinous shells of crustaceans, bone meal, corals, oyster shells, limestone, etc. And not all of them are equally useful. Let's look at specific calcium compounds that are available for purchase.
Calcium-phosphorus imbalance and its consequences in CKD in dogs and cats
Roman Leonard, President of the Russian Scientific and Practical Association of Veterinary Nephrologists and Urologists (www.vetnefro.ru), Head of the Ural Center for Veterinary Nephrology and Urology, Chelyabinsk. Email:
Introduction
Chronic kidney disease (CKD) is an incurable multifactorial disease that steadily progresses in parallel with irreversible processes of destruction in the renal parenchyma and a decrease in the number of their formed elements. And the colossal compensatory reserve of this paired organ is the reason that external manifestations of trouble arise only when more than 3/4 of the nephrons are excluded from the processes of urination, as well as other various endocrine, exocrine and metabolic functions (the result of this is the clinical stage renal continuum1).
And sadly, the diagnosis of CKD today is carried out by veterinarians all over the world most often only in categorical reference to an increase in the level of azotemia. While such a valuable method for diagnosing nephropathy (primarily early), as urine examination, is not carried out at all, its results remain without due attention or are interpreted incorrectly2.
This is all the more surprising, given that already today methods are beginning to be introduced into the practice of veterinary medicine that make it possible to diagnose nephropathies at very early stages, when significant pathological changes are absent even in urine tests. One of these methods is to determine the level of cystatin C. This protein, produced by the nuclear apparatus of most cells, is excreted from the body exclusively by the kidneys, the level of its formation is constant over time in each patient and does not depend on gender, body weight, physical activity, diet and the presence of inflammatory diseases. reactions in the animal (creatinine is very susceptible to this dependence). Along with inulin, determination of glomerular filtration rate (GFR) by the level of cystatin C is today considered the “gold” standard in determining this most accurate test assessing the functional state of the kidneys.
But, returning to azotemia, it should be noted that today such a branch of science as fundamental nephrology (and it, which is quite natural, does not have a clear distinction between veterinary and “human”, since, on the one hand, the structure and functions of the kidneys in mammals (with the possible exception of marine ones) are almost 100% identical3, and on the other hand, research conducted within its framework is inextricably linked with laboratory animals, including cats4), there is no clear evidence that it is urea and creatinine are indeed the leading uremic toxins (the recognition of these substances as such is most likely due only to fashion, dating back almost to the 19th century).
The following facts can be cited as some reasons for this assertion:
- exogenous administration of urea and/or creatinine, even over a long period of time, does not lead to signs of renal failure in dogs and cats, this time;
— the upper limit of normal creatinine level for greyhounds and hounds actively used in work is 220 mmol/l (and this is the third degree of renal failure, according to the IRIS5 classification, classified by the authors as a pathology characterized by a variety of clinical manifestations), that’s two;
— in the practice of most experienced veterinary therapists there have been and/or are patients whose creatinine level is 4–5 or more times higher than the upper limit of normal for the species, according to the IRIS classification, which, however, has a disproportionately small effect on the general well-being of the animal, and “pathology” is usually detected as an incidental finding during examinations carried out for other reasons, these are three;
- and finally, an animal, especially of small size, may even die from the consequences of severe renal insufficiency (subsequently proven histomorphologically) with a completely normal intravital level of azotemia on the eve of death (the creatinine level most likely in this case increased, for example, five times from the original values, which, however, did not prevent this indicator from remaining within the formal norm according to the IRIS classification: 20 mmol/l×5=100 mmol/l).
Some justification can only be that urea and creatinine can in certain cases be markers (but not pathogenesis factors) of renal dysfunction and their accumulation in the body often, although not always, goes in parallel with an increase in the number and/or change in the strength of receptor interactions of other , truly significant, uremic toxins.
At the clinical stage of the renal continuum, the doctor has to deal with a number of serious and practically insoluble problems associated with both the diversity and multidirectionality of clinical manifestations and the inability to use most drugs (including many nephroprotective ones) due to changes in their pharmacodynamics and pharmacokinetics (and the kidneys excrete and/or metabolize most drugs). In addition, there is an inevitable increase in nephrotoxicity even for those drugs that are capable of exhibiting certain nephroprotective properties in animals at the preclinical stage of CKD (for example, ACEIs, CCBs and most ARBs6). It should be noted that the treatment of CKD in dogs and cats at its clinical stage, especially in the current, purely azotemic, concept of CKD, today is reduced only to symptomatic and replacement therapy, the effectiveness of which cannot be high by its definition, and the prognosis of the disease is rapidly changing classified as unfavorable. Moreover, maintaining a high quality of life for patients (again within the framework of the azotemic model) at the clinical stage of CKD, even despite the significant investment of time and material resources on the part of doctors and animal owners, becomes an almost impossible task.
Some minor successes have been achieved by modern veterinary nephrology only in the field of combating renal hypertension (although here everything is far from clear, since most antihypertensive drugs at the clinical stage of the renal continuum acquire certain nephrotoxic properties) and anemia (what happened after the introduction of such a stimulant into practice hematopoiesis, such as dardepoetin alfa, Aranesp7).
Also, certain positive changes have occurred in the fight against hyperphosphatemia. However, both phosphate binders (for example, Renal Candioli, Ipaquitine (containing calcium carbonate and chitosan), and the temporarily discontinued Renalcin8 (containing lanthanum carbonate), and specialized dietary products used for this, are usually prescribed at the clinical stage of the renal continuum, then there is exactly when the effectiveness of their use begins to rapidly decline along with the deterioration of renal function.In the fight against azotemia, no success has been achieved over the past two decades (and it must be admitted that fighting windmills is already a thankless task from the very beginning).
All the more important is the analysis of modern achievements in fundamental nephrology and clinical (applied) human nephrology, which make it possible to reorient the diagnostic vector to new, much more significant problems in the pathogenesis of CKD, and above all to such as mineral-bone disorders. Moreover, one should not think that in this case we are talking only about pathological changes in the skeleton. To one degree or another, all tissues and organs of patients (including the central nervous system) are victims of these disorders, and it is with mineral-bone disorders that most clinical manifestations of the final stages of CKD are currently associated.
Mineral bone syndrome (MBS) in CKD
The concept of MCS, as one of the significant complications during CKD in humans, was proposed and introduced into widespread practice by KDIGO (Kidney Disease Improving Global Outcomes9) in 2006.
The following, expressed to one degree or another in each individual patient, but undoubtedly interrelated manifestations of this pathology are:
- changes in the concentrations of phosphorus and calcium (and to a lesser extent magnesium) in the blood, as well as their normal ratio and localization in cells and tissues (this, among other things, leads to such severe consequences in humans as calcification of blood vessels and soft tissues; however, the severity and the significance of these processes for dogs and cats with CKD is still quite difficult to assess, since such outcomes of this process as strokes and heart attacks are, in principle, extremely rare in dogs and cats);
- increased levels of parathyroid hormone (PTH) (ISS, usually characterized by severe uncontrolled secondary and tertiary (nodular10) hyperparathyroidism) - this is the most significant and common variant of the course of this pathology in cats and dogs);
- decreased synthesis of the active form of vitamin D3 (D-hormone, or calcitriol, is a steroid hormone, traditionally classified as a fat-soluble vitamin);
- severe disruption of metabolic processes and mineralization of bone tissue, leading to such renal osteopathies as fibrous osteitis, adynamic bone disease, osteomalacia with low bone turnover, osteoporosis, as well as a combination of these pathologies; these anomalies are fraught, first of all, not only with multiple pathological bone fractures, but also with the development of severe and poorly controlled bone pain syndrome, inevitably ending in chronic stress, which, in turn, especially in cats, can negate all the efforts of doctors and owners and lead to rapid death of the patient.
Etiopathogenesis of MCS in CKD
Normally, the kidneys, skeletal system and small intestine take part in the regulation of calcium and phosphorus metabolism in the mammalian body. It is well known that these organs are targets for the active action of hormones such as PTH, its functional antagonist calcitonin (CT), as well as calcitriol.
So, acting on the kidneys, PTH:
- stimulates the processes of 1-hydroxylation in the epithelial cells of the proximal tubules, which leads to an increase in the synthesis of calcitriol (other, so-called inactive forms of vitamin D, do not have biological activity), which in turn leads to an increase in the absorption of calcium, and to a lesser extent phosphorus, from food in the intestines;
- reduces the excretion of calcium from the body in the urine by stimulating its reabsorption in the tubules;
- has a pronounced phosphaturic effect.
The effect of PTH on the skeletal system normally causes its renewal, and with a sharp decrease in the level of serum calcium leads to the rapid release of this cation (as well as phosphorus and bicarbonate) into the bloodstream from osteoblasts and osteocytes and indirectly through stimulation of the production of certain cytokines, osteoclasts11. PTH also stimulates the processes of calcium absorption from the intestine, both directly and indirectly, through stimulation of calcitriol synthesis.
In CKD, all areas of phosphorus-calcium homeostasis undergo changes. Moreover, the first pathological changes appear long before the clinical stage of CKD.
The etiopathogenetic mechanisms for the development of MCS in CKD are considered:
- decreased synthesis of the active form of vitamin D3;
- hypocalcemia (both relative, associated with pathological intra- and extracellular redistribution of ionized calcium, and absolute, due to a general decrease in plasma protein-bound deposited in bone tissue and ionized free calcium12 in the body; even transient hypocalcemia almost instantly leads to activation of calcium receptors (CaR) on parathyroid cells, and PTH production increases sharply13);
- decreased excretion of phosphorus by the kidneys;
— an inadequately high response of bone tissue to the action of PTH in the preclinical and early clinical stages of CKD, leading to intensive release from the bone tissue of not only calcium, but also phosphorus (at the clinical stage, on the contrary, bone tissue reacts inadequately and weakly to the action of PTH, which significantly increases the synthesis and strength of receptor interactions of this hormone, transferring it to the category of uremic toxins (the dose-dependent effect in this case is very significant).
However, the leading mechanism for the development of hyperphosphatemia in CKD is associated with a significant decrease in GFR in patients, severe damage to the tubular apparatus of still functioning nephrons (and tubular dysfunctions always aggravate glomerular ones and vice versa, since the nephron structure is both physiologically and partly anatomically closed14) and inability for this reason kidneys remove phosphorus from the body in urine in the volume necessary to maintain homeostasis.
The listed factors are responsible for the irreversible processes of parathyroid hyperplasia and a progressive increase in the level of PTH in the body.
A pronounced intraglomerular decrease in magnesium levels also has a certain stimulating effect on PTH production.
Unlike creatinine, which, when its level in the blood increases, begins to be rapidly and in large quantities eliminated from the body through the gastrointestinal tract (this is one of many reasons that reduces the value of determining the level of renal function by its level), phosphates can leave the body only in the composition of urine (we are, of course, not talking about phosphorus, which transits through the gastrointestinal tract).
Already when GFR decreases by more than 50% (and we should not forget that the first clinical manifestations of kidney disease appear, as a rule, only when this indicator decreases by more than 75%), the serum concentration of phosphorus periodically begins to increase, a positive balance of this is formed element in the body, and the mechanisms of its accelerated elimination come into play.
Initially, thanks to the colossal compensatory capabilities of the kidneys (in this case, primarily their tubular apparatus) and an increase in synthesis:
— fibroblast growth factor 23 (FGF-23, fibroblast growth factor-23): this is a hormonal peptide, the effects of which are realized as a result of binding to the transmembrane protein Klotho, which in this case acts as a coreceptor15 and is located on the membranes of epithelial cells of the proximal tubules), as well as
- PTH (specific receptors for this hormone are also located on tubular cells), the amount of phosphorus in the body can remain within the physiological norm for a long time. This condition, sometimes lasting for years, is today defined as compensated hyperphosphatemia.
But since many of the obvious external symptoms of MCS in CKD are associated primarily with an increase in the amount of PTH (this hormone is now recognized as a universal uremic toxin, directly or indirectly causing the widest range of symptoms of the clinical stage of the renal continuum - from uremic dermatitis and gastritis to renal hypertension and renogenic hypertrophy of the left ventricle ) and FGF-23 (it is classified as a potentially independent uremic toxin, since it has been proven that it has a negative effect on the body of patients with CKD even in the absence of hyperphosphatemia and hyperparathyroidism), many of them begin to appear long before a significant increase in phosphorus levels in the body .
Practicing veterinarians encounter the clinical symptoms of MCS in CKD (primarily this concerns cats, the prevalence of nephropathies in which is many times higher than in dogs) almost every day (deterioration in the quality of skin and coat, focal alopecia, decreased or even complete loss of appetite, pathological bilateral mydriasis (resulting from renal hypertension and hyperparathyroidism), intermittent vomiting, asthenia, weight loss, renogenic cardiopathy, etc.). But, unfortunately, these abnormalities are usually interpreted as not related to CKD. Although, to limit the range of pathologies possible in a patient and even verify the diagnosis, as a rule, a general clinical urine test and analysis of scientific data (based primarily on histomorphological studies of the renal parenchyma) on the prevalence of certain nephropathies in large populations of cats and dogs are sufficient.
The point of application of the action of the hormonal peptide FGF-23 synthesized in bone cells is primarily the tubular apparatus of the nephron, where it, firstly, reduces the reabsorption of phosphorus, thus increasing its amount in the final urine, and, secondly, inhibits the activity of the enzyme 1-hydroxylase, thereby inhibiting the synthesis of calcitriol. The latter is justified from the point of view of the pathogenesis of MCS in CKD, since, together with calcium, D-hormone stimulates the absorption of phosphorus in the intestine, and the more intense the more severe the stage of CKD. Today it has been proven that it is FGF-23, together with Klotho proteins, that serve as the first significant line of defense for the body against increasing hyperphosphatemia. And the number of both biologically active molecules, as well as the strength of their receptor interactions, increases significantly already in the early preclinical stages of CKD. Therefore, increased levels of FGF-23 and/or Klotho protein are now considered as a precursor to the presence and progression of MCS in patients in the early preclinical stages of CKD. However, the clinical stage of CKD, on the contrary, is usually characterized by a decrease in the level of the transmembrane form of Klotho proteins (but not FGF-23) due to damage to the proximal tubules where it is formed.
Another target for FGF-23 is the cells of the parathyroid glands themselves. By acting on them, FGF-23 initially both stimulates the active production of PTH and initiates and maintains the processes of their proliferation and hyperplasia. This, in turn, leads to such irreversible consequences as uncontrolled secondary and tertiary hyperparathyroidism. Subsequently, as the severity of CKD worsens, high concentrations of FGF-23 cause the sensitivity of the parathyroid glands to both ionized calcium and PTH itself to be significantly reduced, which leads to even greater production of this universal uremic toxin.
Subsequently, the inevitable progression of destruction processes in the renal parenchyma leads not only to a significant decrease in GFR, but also to the formation of resistance to the action of FGF-23, associated with a pronounced deficiency in the renal production of the transmembrane form of the Klotho protein (no receptor - no action of the signaling molecule). All these processes further aggravate the severity of decompensated hyperphosphatemia and intoxication caused by PTH in the clinical stage of CKD.
A certain role in the progressive increase in the level of PTH in the body is played by a decrease in the synthesis of D-hormone in the proximal sections of the tubules (and severe damage, especially to the proximal sections, of the nephron tubular apparatus is an integral part of the pathogenesis of CKD, regardless of the primary form of nephropathy that led to it). PTH stimulates the processes of 1-hydroxylation of inactive forms of vitamin D2 into calcitriol. On the other hand, D-hormone, acting on its receptors on the cells of the parathyroid gland, inhibits the production of PTH.
Also, a decrease in the level of calcitriol leads to a decrease in calcium absorption in the intestine, the development of hypocalcemia and, accordingly, to additional stimulation of PTH production.
However, calcitriol-dependent pathways for stimulating PTH production in dogs and cats, at least from a theoretical point of view, cannot be considered leading, since the synthesis of this steroid occurs not only in the kidneys, but also in the cells of the lymphohemapoietic system and in osteocytes. And vascular smooth muscle cells produce not only 1-hydroxylase, but also receptors for vitamin D3, through which most of its biological effects are realized. Moreover, the significance of the so-called alternative pathways for calcitriol formation appear to increase with increasing severity of tubulointrestial lesions.
It should also be taken into account that the introduction of active forms of vitamin D3 (this also includes a synthetic analog of calcitriol, such as alfacalcidol) in animals with CKD can lead to severe D-hormone intoxication (and the therapeutic index of these drugs is very narrow) and additional exogenous aggravation of the severity hyperphosphatemia. The latter phenomenon is primarily due to the fact that active forms of vitamin D3 in CKD equally stimulate an increase in intestinal absorption of both calcium and phosphorus, especially if its intake is not limited dietaryly and/or using phosphate binders.
Another possible mechanism leading to an increase in the level of PTH in MCS is a decrease in the sensitivity of bone tissue cells to its calcemic effect, which also occurs against the background of hyperphosphatemia and hyperparathyroidism (thus, closing the vicious circle of etiopathogenesis of MCS in CKD). This same process may also stimulate the accumulation in the body of various toxic metabolites and inflammatory and oxidative stress factors inherent in the clinical stage of the renal continuum. In addition, the rate of bone resorption at the clinical stage of CKD has certain limits, beyond which even a significant increase in PTH concentration will not lead to an additional increase in the release of calcium into the blood. It is possible that these processes will even have the opposite effect, the essence of which is to suppress the functional (metabolic) activity of the bone tissue cells themselves and reduce the flow of ionized calcium into the blood.
Moreover, it should be taken into account that against the background of hyperparathyroidism and hyperphosphatemia (even compensated), active exogenous administration of calcium will not only not benefit the bone tissue, but can also lead to irreversible processes of soft tissue calcification. Also, such actions will not lead to a decrease in the level of PTH itself, since by the clinical stage of CKD the parathyroid glands are already in a state of secondary and tertiary hyperpalasia and even complete normalization of the phosphorus-calcium balance will not reduce the intensity of synthesis and excretion of prohibitively high doses of PTH. Secondary and tertiary hyperparathyroidism is an irreversible process, and the problem can only be eliminated surgically, which is not possible in cats and dogs today, at least not in widespread surgical practice.
Conclusion
Thus, a parallel increase in the severity of hyperphosphatemia and hypocalcemia, a decrease in the sensitivity of bone tissue cells to the action of PTH, disturbances in the receptor interactions of PTH itself and receptors located on the cells of the parathyroid glands and (to some extent) a decrease in the level of calcitriol are factors responsible for the formation of uncontrolled secondary and tertiary hyperparathyroidism in dogs and cats with CKD and the development of the vast majority of clinical manifestations of the clinical stage of the renal continuum.
With a high degree of probability, it can be assumed that it is phosphorus retention in the body, which inevitably ends in compensated and decompensated hyperphosphatemia, that is one of the prevailing factors in the development of uncontrolled secondary and tertiary hyperparathyroidism and, consequently, the appearance of the overwhelming number of clinical symptoms of MCS in CKD in dogs and cats. This circumstance is a good reason for starting “renal” diet therapy containing the minimum possible amount of phosphorus and the use of phosphate binders (calcium carbonate, sevelamer, lanthanum salts16) at the preclinical stage of CKD. And since the level of proteinuria closely correlates with the severity of MCS, a persistent increase in even just this indicator in a patient by more than 0.01 g/l (especially in combination with a reduction in urine density and agranolucyturia) is the basis for starting measures to reduce the intake of phosphorus into the body. At the clinical stage of the renal continuum, when animals have no or distorted appetite for a number of reasons, the selection of dietary therapy becomes significantly more complicated (if possible at all), and its meaning is largely neutralized, since even the complete exclusion of phosphorus from the diet (which, of course, is impossible , especially in the case of such an obligate predator as cats) will not lead to a decrease in the level of intoxication caused by uncontrolled secondary and tertiary hyperparathyroidism.
Determining the level of PTH in blood serum in dogs and cats today is associated with certain difficulties, since laboratory reagents used to verify this indicator in humans are not complementary to animals, and veterinary test systems have not yet become widespread. Therefore, although indirect, but easily accessible tests are so important to assess the significance of MCS in CKD. These include, for example, alkaline phosphatase (ALP). This enzyme takes an active part in the processes of decalcification of bone tissue in MCS, and therefore its level increases significantly in patients, especially at the clinical stage of CKD.
Since the reabsorption of phosphorus in the tubules is directly coupled with the reabsorption of sodium via the Na/P cotransporter, a significant dietary restriction in the consumption of table salt can lead to an aggravation of the severity of hyperphosphatemia in patients with CKD due to a significant increase in the number and activity of the transport mechanisms of elements of this pair. It should also be taken into account that dietary NaCl stimulates the absorption of calcium in the intestine from food. In addition, a decrease in sodium levels in the body leads to an increase in the activity of the (already hyperactivated due to nephropathy) renin-angiotensin-aldosterone system.
Notes
1 The renal continuum is the period of time from the appearance of the first histomorphological changes in the renal parenchyma, usually diagnosed using light and/or electron microscopy, until the patient dies from the consequences of renal failure.
2 For example, the author of the article constantly has to deal with the opinion of practicing doctors who classify non-selective nephrotoxic proteinuria as a normal variant (although all the i’s in this matter can be easily dotted based on banal physiological knowledge about the structure and functional characteristics of the filtration barrier and tubular nephron apparatus). Moreover, several times I even had to convince my colleagues that increasing the patient’s proteinuria level to the so-called. “norms”, although quite feasible (introducing most nephrotoxic poisons, such as ethylene glycol, to an animal or a person, can help with this), but somewhat redundant, from the point of view of modern nephrology, is a task.
3 A significant confirmation of this is the fact that all medications intended for the treatment of kidney diseases in dogs and cats, as well as the purposes for their use, are borrowed from human medicine.
4 For example, it is generally accepted that the causes of the appearance and nature of the course of such nephropathy as polycystic kidney disease are almost identical for humans and cats, which allows us to regard (and use) these animals as the most suitable object for scientific research aimed at prevention and control this serious pathology.
5 https://iris-kidney.com
6 Angiotensin-converting enzyme inhibitors, calcium channel blockers and angiotensin receptor blockers.
7 The introduction of “human” erythropoietins to cats and dogs rapidly leads (as a rule, after the second injection) to the formation of autoantibodies in the body, which not only almost completely neutralizes their hematopoietic effect, but also ends in additional hyperactivation of the own immune system (and excessive autoimmune reactions are one of the significant factors in the formation and progression of CKD).
8 The author has information that she transferred the rights to produce Renalcin to another pharmaceutical company.
9 Initiative to Improve Global Outcomes in Kidney Disease. KDIGO is one of the most authoritative scientific and practical associations specializing in the field of human nephrology.
10 Nodular.
11 Osteoclasts, strictly speaking, do not belong to bone cells, but are of monocyte origin and belong to the macrophage system. These are large multinucleated cells involved in the processes of bone tissue destruction.
12 The amount of ionized calcium (Ca2+) in the blood determines the completeness of all biological effects of this cation.
13 Similar calcium receptors are located on the C-cells of the thyroid gland, which produce the PTH antagonist calcitonin, as well as on the cells of the brain and kidneys.
14 One of the walls of the distal tubule in the area of the so-called. The macula densa merges with the juxtaglomerular and extramesangial cells (these structures are generally located between the afferent and efferent arterioles) of the glomerulus propria. Such close contact of different parts of the nephron is necessary for the constant exchange of information about the composition of secondary urine, regulation of intraglomerular blood flow, changes in the porosity of the filtration barrier layers, etc.
15 Coreceptor is an additional receptor that appears on the surface of the cell in the event of a particular biological need. By binding to a signaling molecule (for example, the Klotho protein binds to the FGF-23 molecule on the cell surface of the tall columnar epithelium of the proximal tubule), the coreceptor repeatedly enhances the already existing effects (in this case it will be a phosphaturic effect and suppression of D-hormone synthesis).
16 Aluminum salts, recommended by some authors, are unacceptable to use as phosphate binders, since they cause aluminum intoxication in patients, significantly aggravate the destruction of bone tissue (aluminum begins to replace calcium in osteoblasts and osteoclasts) and thereby increase the severity of MCS in CKD.
Literature
1. Block G. A., Raggi P., Bellasi A. et al. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int.2007; 71:438–441.
2. Craver L., Marco MP, Martinez I. et al. Mineral metabolism parameters throughout chronic kidney disease stages 1–5-achievement of K/DOQI target ranges. Nephrol. Dial. Transplant. 2007; 22: 1171–1176.
3. Denda M, Finch J, Slatopolsky E. Phosphorus accelerates the development of parathyroid hyperplasia and secondary hyperparathyroidism in rats with renal failure. Am J Kidney Dis 1996; 28(4):596–602
4. Fliser D, Kollerits B, Never U et al. Fibroblast growth factor 23 (FGF-23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol 2007; 18(9):2600–2608
5. Foley RN, Parfrey PS, Sarnak MJ Clinical epidemiology of cardiovascular disease in chronic renal disease. Am. J. Kidney Dis. 1998; 32:S112–S119.
6. Fucumoto S. Physiological regulation and Disorders of Phosphate metabolism - Pivotal Role of Fibroblast Growth factor-23. Inter. Med. 2008; 47: 337–343.
7. Galitzer H, Ben-Dov IZ, Silver J et al. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int 2010; 77(3):211–218
8. Horl, WH The clinical consequences of secondary hyperparathyroidism: focus on clinical outcomes. Nephrol Dial Transplant. - 2004. - Vol. 19 (Suppl 5). — V2–8.
9. Isakova T, Gutierrez O, Shah A et al. Postprandial mineral metabolism and secondary hyperparathyroidism in early CKD. J Am Soc Nephrol2008; 19(3):615–623
10. Isakova T, Wahl P, Vargas GS et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 2011; 79 (12): 1370-1378
11. Jean G. High levels of serum FGF-23 are associated with increased mortality in long haemodialysis patients. NDT 2009 24(9); 2792–2796.
12. Makoto K. Klotho in chronic kidney disease — What's new? Nephrol. Dial. Transplant. 2009; 24(6): 1705–1708.
13. Maschio G, Tessitore N, D'Angelo A et al. Early dietary phosphorus restriction and calcium supplementation in the prevention of renal osteodystrophy. Am J Clin Nutr 1980; 33(7):1546–1554
14. National kidney foundation. K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. Am. J. Kidney Dis. 2003 (suppl. 3); 42:S1–S202.
15. Razzaque MS Does FGF23 toxicity influence the outcome of chronic kidney disease? Nephrol. Dial. Transplant. 2009; 24(1): 4–7. 29. Gutierrez OM Fibroblast Growth Factor 23 and Left Ventricular Hypertrophy in Chronic Kidney Disease. Circulation. 2009; 119:2545–2552.
16. Sitara D. Genetic ablation of vitamin D activation pathway reverses biochemical and skeletal anomalies in Fgf-23-NULL animals. Am. J. Pathol. 2006; 169:2161–2170.
17. Ermolenko V.M. Chronic renal failure. Nephrology: national guidelines. Ed. ON THE. Mukhina. M.: GEOTAR-Media. 2009; 579–629.
18. Rozhinskaya L. Ya. Secondary hyperparathyroidism and renal osteopathies in chronic renal failure. Journal "Nephrology and Dialysis" Vol. 2, 2000, No. 4
SVM No. 1/2016
Rate material
Like Like Congratulations Sympathy Outrageous Funny Thoughtful No words
Vitamins for cats with calcium
Vitamins with calcium for cats may be needed in the following cases.
- The cat's food is depleted in vitamins and minerals (for example, when eating inferior food or boiled food).
- For some reason (for example, internal pathologies), the animal has a problem with the absorption of microelements - then during treatment the body needs to be supported with vitamins and minerals.
- During a period of active growth, stress and increased stress on the body.
- For some diseases of the musculoskeletal system and other pathologies.
- Veterinarians advise animals on a natural diet to take 2 courses per year. Despite the fact that a proper natural diet does not need to be supplemented with vitamins and minerals, owners often fail to balance it 100%, which is why pets require periodic support with supplements.
Among the good vitamin-mineral complexes that include calcium for cats are the following: Beaphar (Netherlands), Canina (Germany), 8in1 from Brewers (USA), Nutri Vet (USA), Gimpet (Germany), Anivital ( Germany), Bayer (Germany), Dreamies (USA).
These complexes are more expensive than others, but cheap vitamin and mineral preparations are absorbed much worse, so it’s not worth saving.
Vitamins are sold in the form of tablets, powders and pastes. Calcium paste is suitable for kittens and elderly animals without teeth. Most cats calmly eat pills. In powder form, vitamins and minerals are convenient to add directly to food.
How and when to give your cat calcium
The daily dose of calcium is calculated based on the amount of phosphorus consumed with meat. To do this, you need to know how many grams and what kind of meat the cat eats. Calcium for adult cats is calculated based on a ratio of 1.2:1 or at least 1:1. Calcium for kittens is calculated based on a ratio of 1.4:1. Typically, 60-100 mg of calcium per 1 kg of animal weight is recommended, unless otherwise specified by a veterinarian.
Calcium is supplied with food. At the same time, it is better to give the daily dose of calcium not at once, but in 2-3 doses, because Calcium is well absorbed in small portions. But if you give all the calcium in 1-2 doses, then do it in the afternoon, ideally before bed. This is due to the metabolic characteristics of cats.
Some nutritionists recommend giving different amounts of calcium every day: on one day - more than needed, on another - less, and on the third, “forget” altogether. In this way, they believe, we reproduce the natural diet of a wild cat, which does not consume the same amount of food day after day. But then the amount of meat should not be equal, because the calcium-phosphorus combination in a cat’s natural food is always maintained: the cat either eats the caught carcass with bones and meat, or does not eat it, or eats one, or 5, depending on how much game there is will catch. Thus, the ratio of calcium and phosphorus is always maintained. If you give different amounts of food, then on some days the cat may overeat, and on others it may feel hungry. We consider it inappropriate to arrange a food “swing”.
But still, with periodic insufficient calcium intake, nothing bad will happen. If an adult cat is constantly on a calcium-depleted diet, the critical period is considered to be 2 months: if all this time the cat received almost no calcium, consequences associated with the development of various pathologies occur.
With a constant excess intake of calcium, the cat’s body reduces absorption, i.e., generously giving this element can cause the opposite effect.
Deficiency and consequences
At each stage of life, a cat should receive the required amount of calcium appropriate to its age and needs. Without calcium, processes such as the transmission of nerve impulses and muscle contraction are not possible.
Proper calcium intake – in the right amount and at the optimal period. If a cat lacks a microelement, this immediately affects its health, and the consequences are different at each age. For example, if a kitten lacks calcium, then the situation is aggravated by pathologies in skeletal development. Lack of calcium in a cat in adulthood and old age leads to brittle bones - the possibility of fractures increases. Adult pets feel a lack of this element in the fifth month - that is, five months after the body experiences a shortage. In kittens this happens much faster.
It also happens that a cat has high calcium - what to do in this case? Of course, stop giving your cat calcium. It should be remembered that pets at different periods of life require an increased rate of this microelement - especially at a young age, during lactation. Excessive calcium content in a cat’s body is fraught with bone pathology and osteochondrosis.
It is important to adhere to the consumption norm. Is your cat lacking calcium? Use an approximate calculation of consumption - only a veterinarian will provide more accurate information.
- kittens - 440 mg per 1 kg of weight ;
— adult cats – 45 mg per 1 kg of weight .
For example, an adult cat weighing 4 kg should receive 180 mg of calcium per day .